A fractal is a geometrical pattern that tends to repeat itself regardless of the scale at which one observes it. The complex shape of a fractal often gives an impression of the endless repetition of a pattern.
Fern leaves, pine cones, romanesco broccoli, and succulent leaves are all examples of fractal. However, all such previously identified fractals exist on a macroscopic level.
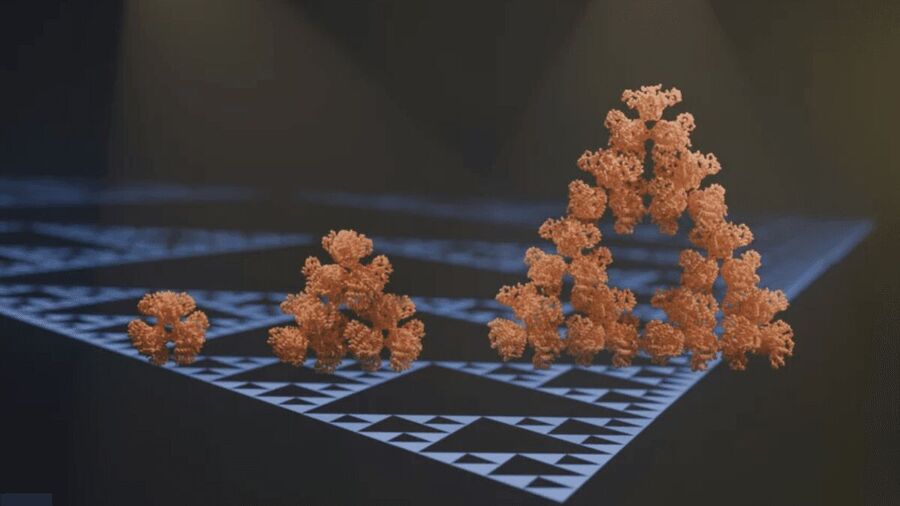
This is the first time researchers have found molecular fractal in citrate synthase, an enzyme found in cyanobacteria Synechococcus elongatus.
"We stumbled on this structure completely by accident and almost couldn't believe what we saw when we first took images of it using an electron microscope," Franziska Sendker, lead researcher and a scientist at Max Planck Institute of Terrestrial Biology, said.
The fractal molecule appeared unexpectedly
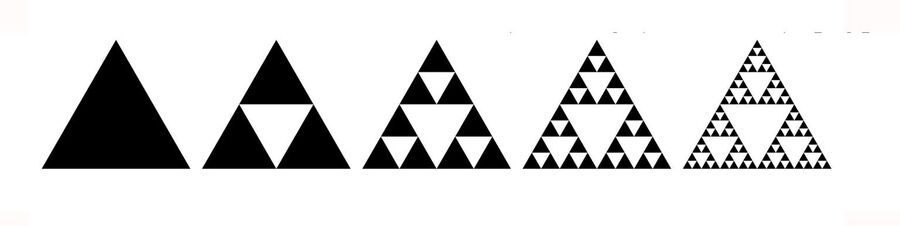
During their study, the researchers noticed that citrate synthase spontaneously took the shape of the Sierpiński triangle, a well-known fractal pattern.
Sierpiński triangle is formed by repeatedly cutting out smaller equilateral triangles from a large equilateral triangle. The continuous and infinite removal of smaller triangles produces a geometric shape that comprises an endless number of smaller triangles.
The study authors were busy observing proteins in different types of cyanobacteria under an electron microscope. This is when they noticed that citrate synthase was self-assembling into beautiful triangles with triangular voids in them.
"We report the discovery of a natural protein, citrate synthase capable of forming Sierpiński triangles in dilute aqueous solution at room temperature," the researchers note.
The pattern appeared suddenly and immediately disappeared in all except one bacterium, S. elongatus.
It was an unexpected finding because no other known bacterial enzyme or protein demonstrates such behavior.
"It popped into existence very suddenly and was then almost immediately lost again by a few different versions of bacteria, and only stuck around in this one cyanobacterium, which makes our discovery of it almost more bizarre because our chances of finding it were basically near zero," Georg Hochberg, senior study author told New Scientist.
Peculiar features of the first fractal molecule
The complex geometry of fractals especially at a molecular scale makes it tricky to study them.
For instance, "Image averaging techniques kept getting confused by the fact that the smaller triangles can be substructures of larger triangles," Jan Schuller, one of the researchers and an expert in structural biology at the University of Marburg, said.
During the study, even "The algorithm kept homing in on these smaller triangles instead of seeing the larger structures they were part of," Schuller added. However, thanks to electron microscopy, the researchers could finally study the fractal structure.
The observation revealed that the fractal protein's assembly is different from the assembly of regular proteins. Generally, all protein chains follow the same arrangement in a regular protein assembly.
In contrast, the formation of the Sierpiński triangle introduced an unusual asymmetry in citrate synthase. The large triangular voids made the fractal protein chains arranged in different positions.
However, what's the effect of this geometry on the bacterium? To find the answer to this question, the researchers cultured S. elongatus cells after blocking the citrate synthase assembly. Interestingly, the cells grew normally without exhibiting any change.
So does that mean fractal molecules only produce beautiful and complex patterns, and they don't have any role in biology or evolution?
Well, it's too early to say that but hopefully, future studies will shed more light on the role these structures play in nature.
The study is published in the journal Nature.



WTF. They have image averaging software corrupt the data (images) before they even lay eyes on it. How is that science? Well, maybe it is if they work for Phizer, Big AGRA or the MIC.
Hmm...